Our Research
Building biologically informed therapy selection systems and new therapies for patients with blood cancer using advanced multi-omic technologies
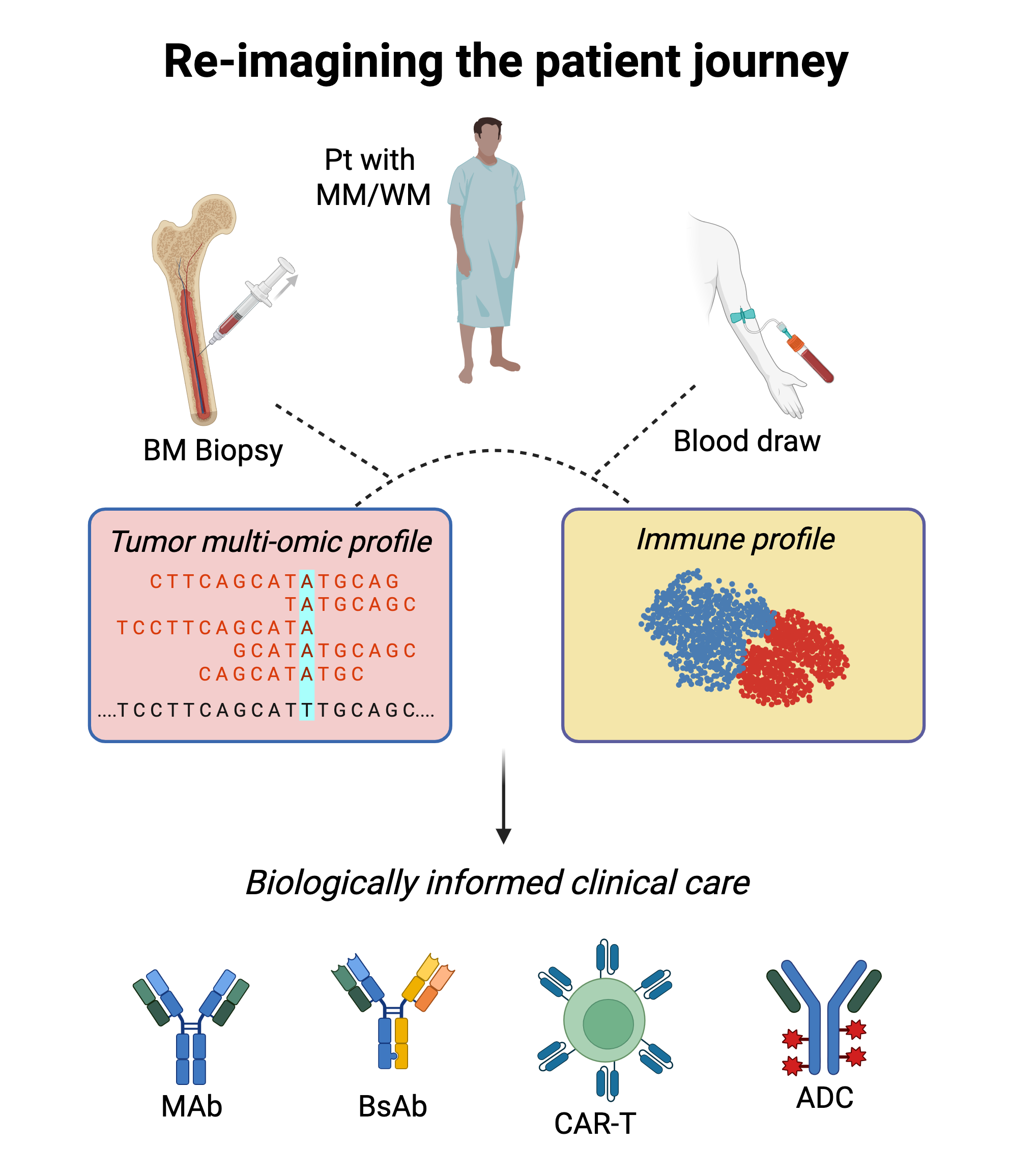
Despite significant progress, it remains challenging to accurately predict who will develop blood cancer and which patient will respond to what therapy. Most patients with Multiple Myeloma or Waldenstrom’s Macroglobulinemia will receive the same treatment, which in the case of Myeloma typically targets all plasma cells indiscriminately, whether they are normal or malignant. By using advanced multi-omic technologies to measure and decode tumor biology and the state of the patient’s immune system, the RSP lab aims to usher in an era of biologically informed clinical decision-making for patients with blood cancer.
Decoding tumor evolution, spread & response to therapy
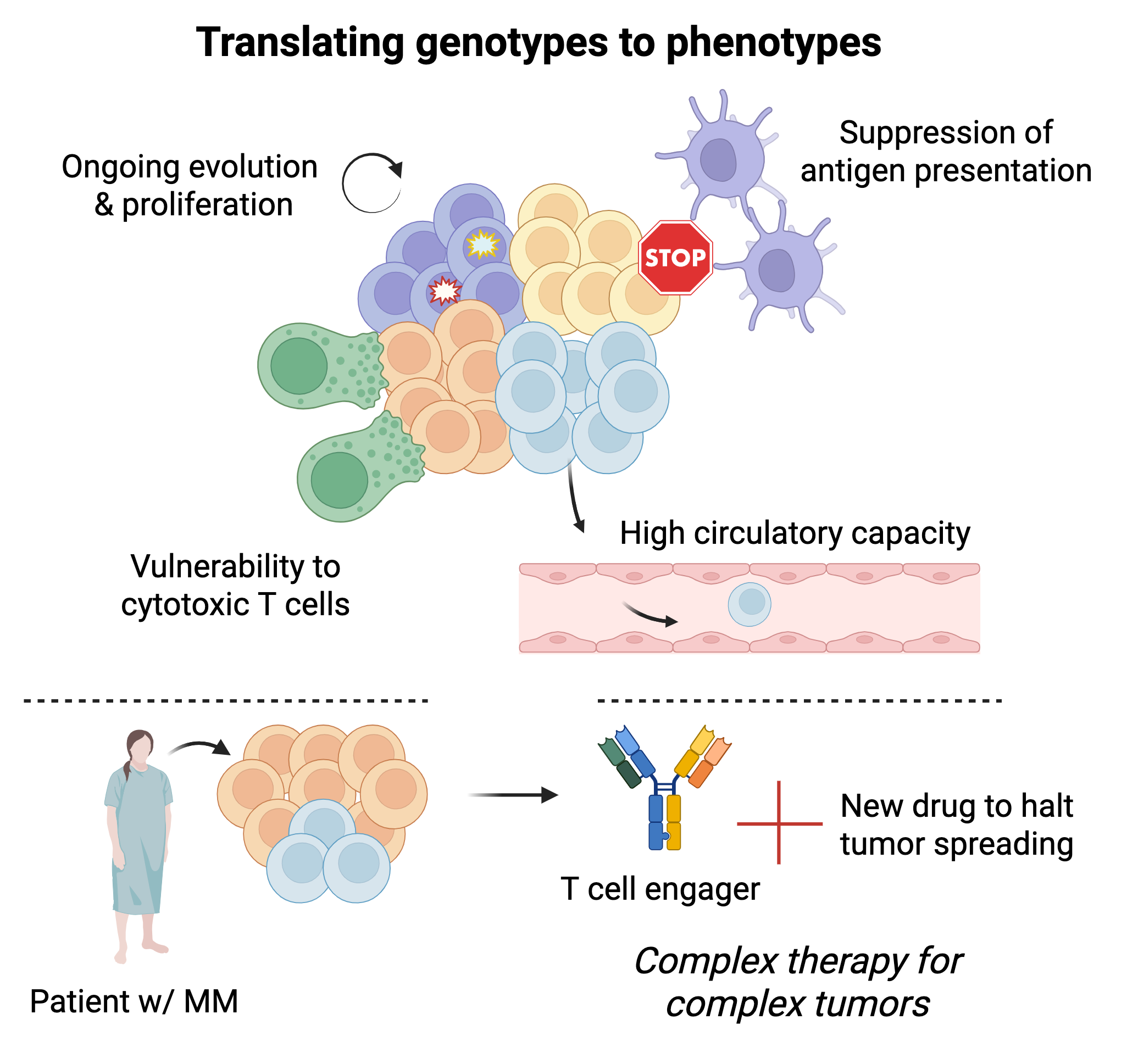
Not all cancer cells will act or react the same way. What makes certain cancer cells more likely to proliferate, spread, evade the immune system, and survive treatment? We use single-cell multi-omic & lineage tracing technologies to study how genomic and transcriptomic abnormalities influence phenotype and behavior and identify clinically relevant biological programs. We then leverage this knowledge to (i) develop and deploy next-generation clinical-grade assays to help guide clinical care, and (ii) inform drug development.
See our publications:
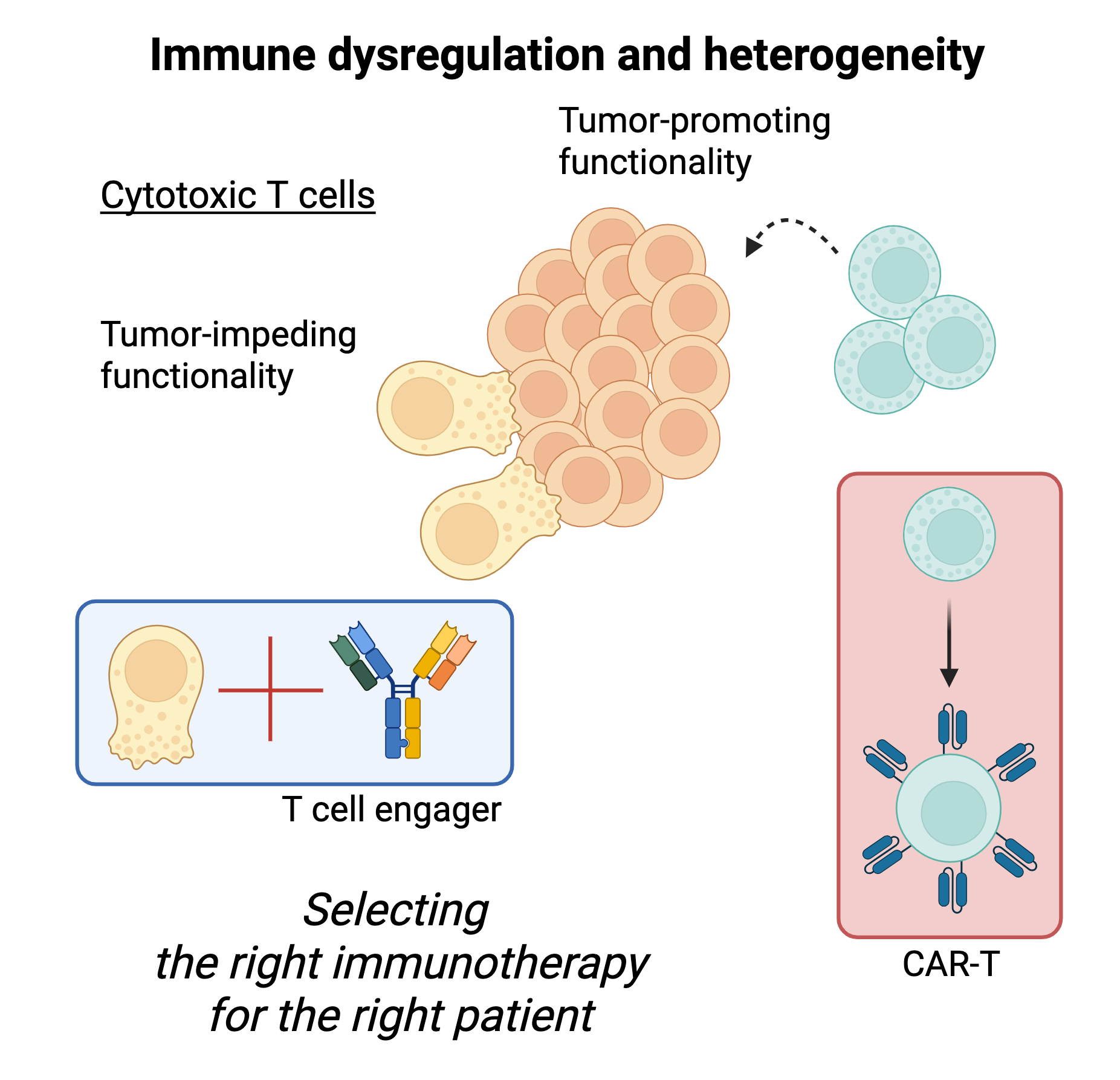
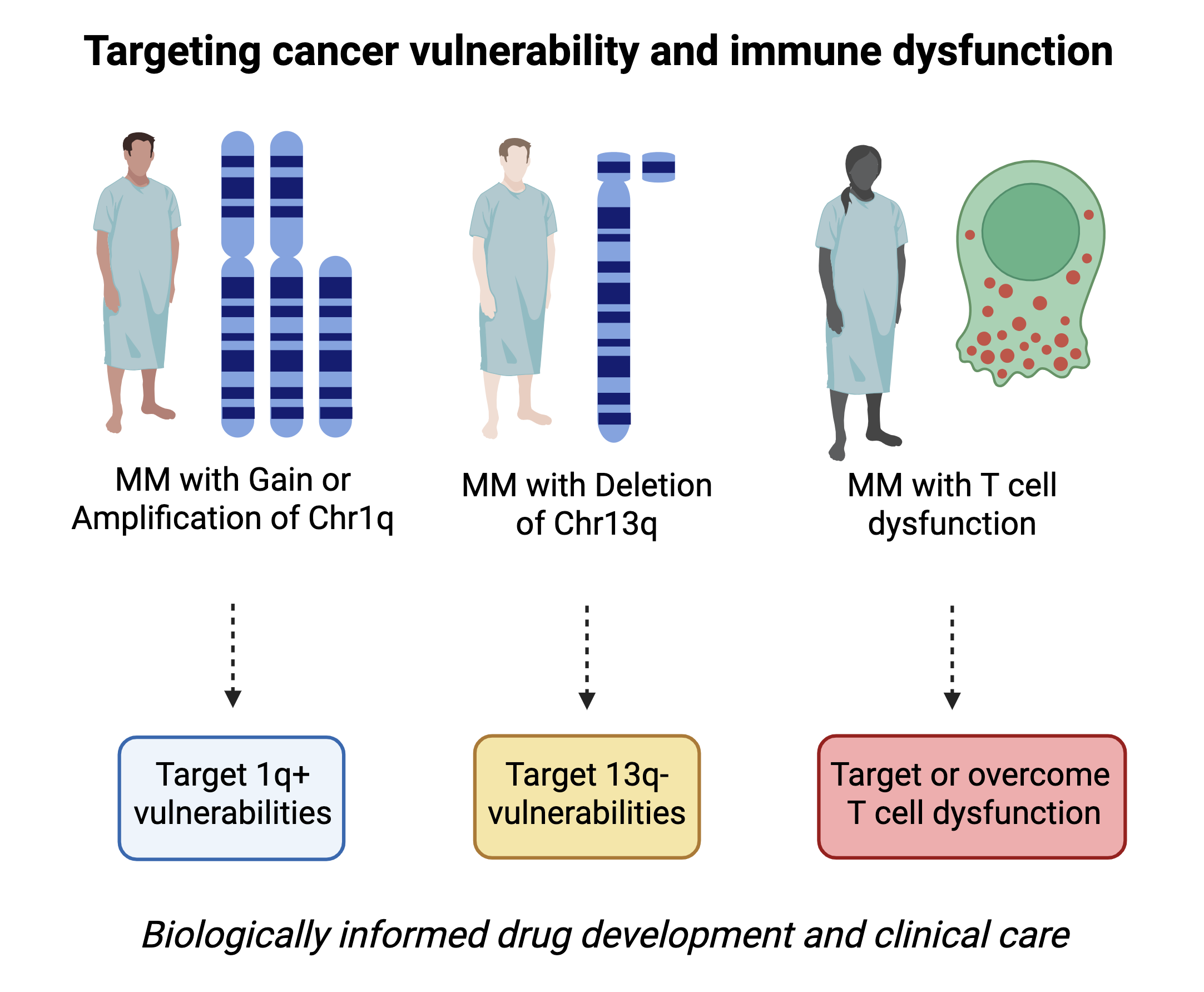
Tumor-intrinsic abnormalities tell only a piece of the story. The state of the patient’s immune system at diagnosis, the quality of the immune responses launched during treatment, and the normalization of the immune system post-therapy may all play a key role in determining outcomes for patients with blood cancer, such as myeloma. And like cancer cells, immune cells exhibit heterogeneous states and responses to therapy. Using single-cell multi-omic technologies, we comprehensively characterize cancer-related immune dysregulation and study how immune responses form and resolve over time during and after therapy. We then develop immune biomarkers that can inform therapy selection in the clinic and identify new opportunities for immunotherapy development.
Deciphering immune dysregulation & immune response to therapy
Identifying new opportunities for precision therapy & immunotherapy
Although several new drugs have been approved, myeloma therapy remains not biomarker-driven. This is due to myeloma’s genetic heterogeneity, resulting in few recurrent genomic alterations, most of which involve so far undruggable targets or are arm-level and chromosome-level alterations that are challenging to study and target. Combining single-cell multi-omic technologies with genetic dependency screens and drug screens, we identify new opportunities for drug development or repurposing that are tied to specific abnormalities in patients with blood cancer. In doing so, we aim to expand and optimize the therapeutic armamentarium for use within a biologically informed clinical decision-making framework.